
Cesium and germanium: According to Pauling scale, the electronegtaivity of cesium and germanium is 0.79 and 2.01 respectively.

Since, the difference is more than 2.0 thus, the bond formed is ionic.ģ. Strontium and chlorine:According to Pauling scale, the electronegtaivity of strontium and chlorine is 0.95 and 3.0 respectively. Since, the difference is less than 2.0 thus, the bond formed is not ionic.Ģ. Carbon and oxygen: According to Pauling scale, the electronegtaivity of carbon and oxygen is 2.5 and 3.5 respectively. In the Pauling scale, if the electronegativity difference between atoms of two elements is more than 2.0, then the bond formed between them is ionic.ġ.
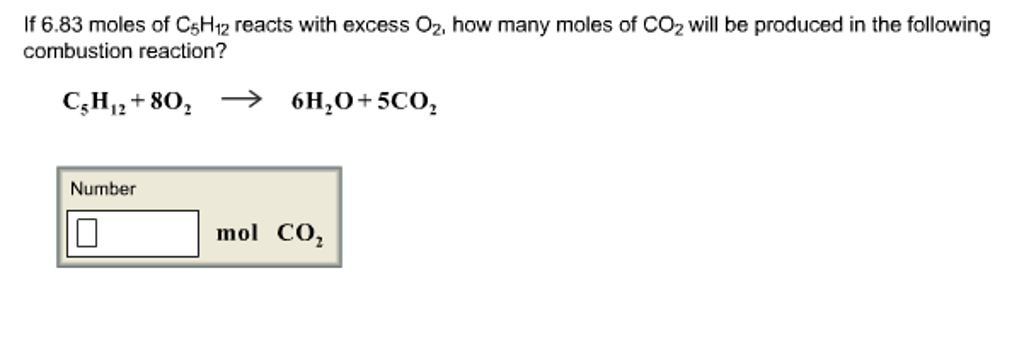
More is the difference, stronger will be the ionic compound. Thus, atoms of two elements having difference in electronegativity will form ionic compound.

#"For H: " (1.5765color(red)(cancel(color(black)("moles"))))/(0.78868color(red)(cancel(color(black)("moles")))) = 1.Ionic bond is formed by complete transfer of electron/s by an atom of electropositive element to an electronegative element. #0.78868color(red)(cancel(color(black)("moles CO"_2))) * "1 mole C"/(1color(red)(cancel(color(black)("mole CO"_2)))) = "0.78868 moles C"#įinally, to find the mole ratio that exists between carbon and hydrogen in the hydrocarbon, divide these values by the smallest one SInce every mole of carbon dioxide contains 1 mole of carbon and 2 moles of oxygen, it follows that the reaction also produced Now, you know that every mole of water contains 2 moles of hydrogen and 1 mole of oxygen, which means that the reaction produced This means that you can use the number of moles of water and carbon dioxide, respectively, to determine how many moles of carbon and of hydrogen were originally present in the hdyrocarbon. Likewise, all the hydrogen that was initially a part of the hydrocarbon is now a part of the water. This tells you that all the carbon that was initially a part of the hydrocarbon will now be part of the carbon dioxide. Notice that the products of this combustion reaction are carbon dioxide, #"CO"_2#, and water, #"H"_2"O"#. The key here is to realize that you're dealing with a hydrocarbon, that is, a compound that contains only carbon and hydrogen.


 0 kommentar(er)
0 kommentar(er)
